Adherus AutoSpray and Adherus AutoSpray ET Dural Sealant
Overview
Adherus AutoSpray and Adherus AutoSpray ET Dural Sealants are indicated for use in patients who are 13 years of age and older, as an adjunct to standard methods of dural repair, such as sutures, to provide watertight closure during cranial procedures.
View the contraindications here.
Demonstrated to be 99.1% effective in preventing CSF leaks through 14 days of post-operative follow-up.1
Adherus AutoSpray and Adherus AutoSpray ET Dural Sealant set-up instructions from Stryker CMF on Vimeo.
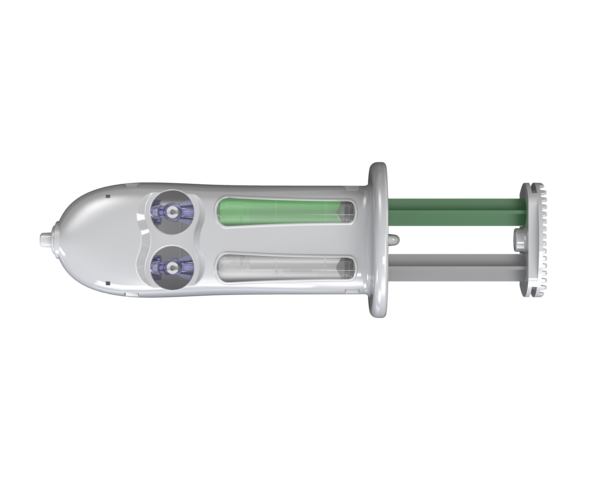
Adherus AutoSpray
Features and benefits:
-
Maintains burst pressure strength above physiological intracranial pressure in an in vitro model2
-
Absorbs over approximately 90 days3
- Pre-assembled applicator
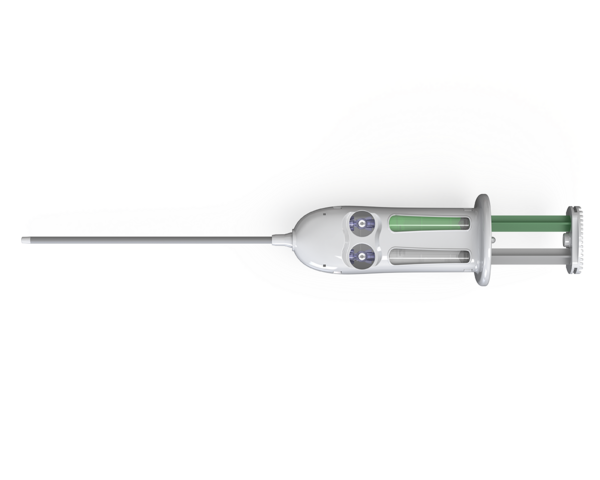
Adherus AutoSpray ET
Features and benefits:
-
Maintains burst pressure strength above physiological intracranial pressure in an in vitro model2
-
Absorbs over approximately 90 days3
- Extended tip applicator
Contraindications:
Adherus AutoSpray and Adherus AutoSpray ET Dural Sealant should not be used in confined anatomical spaces where nerve compression is of concern. The hydrogel may swell by up to 13% of its size in any dimension or 46% by volume after application.
Pivotal trial results:
A prospective, randomized, controlled, multicenter pivotal trial was conducted to evaluate the safety and effectiveness of Adherus AutoSpray Dural Sealant. The primary endpoint of this study was a composite evaluation of the safety and effectiveness of Adherus AutoSpray Dural Sealant (n=124 subjects) when compared to an active control (n=126 subjects). The endpoint results were based on the number of subjects who were free from intra-operative CSF leakage from dural repair after up to two applications of sealant during the Valsalva maneuver, CSF leak/pseudomeningocele during the 120-day follow-up period and unplanned retreatment of the original surgical site within 120 days post-surgery. The overall success rate for the complete case analysis was 91.2% in the Adherus group compared to 90.6% in the control group. Adherus was found to be non-inferior to the control with the non-inferiority margin of 10% (p = 0.005). In the early post-surgical period when the sealants are expected to function, the overall success rate at the 14-day follow-up on subjects who completed the visit was 99.1% in the Adherus group compared to 95.0% in the control group. In addition, the overall success rate at the 45-day follow-up on subjects who completed the visit was 96.6% in the Adherus group compared to 91.9% in the control group. There were no unanticipated adverse device effects. Among the subjects treated with Adherus AutoSpray Dural Sealant, there were no device related deep wound infections and no cases of meningitis. The type and rate of adverse events observed in this study are consistent with the complexity of the surgical procedure and the co-morbid condition of the treated subjects.1
References:
1: Tew, Jr JM, Strong MJ, West GA, et al. A Pivotal Randomized Clinical Trial Evaluating the Safety and Effectiveness of a Novel Hydrogel Dural Sealant as an Adjunct to Dural Repair. Operative Neurosurgy. 13:204-212, 2017.
2: van Doormaal T, Kinaci A, van Thoor S, et al. Usefulness of Sealants for Dural Closure: Evaluation in an In Vitro Model. Operative Neurosurgery. 15(4): 425-432; 2018.
3: Data on file with Stryker
CMF-WC-40_Rev. 2_19254